
ETi 400 High Efficiency Gas Pool Heater TradeGrade
Engineering. David. Etukudo. Share. At atmospheric pressure, gasoline has an initial boiling point of 95 °F (35 °C) and a final boiling point of 395 °F (200 °C). This wide range is due to its variety of blends which alter its boiling point value. Also, pressure is another factor that alters gasoline's boiling point.

ProShares Ultra Bloomberg Natural Gas (BOIL) Stock Surges on Increasing
Joined 17 years ago. 14,019 Posts. With the assistance of Google, I found that the boiling point of ethanol is 173.1F - but the boiling point of gasoline isn't as clear, with that boiling point being between 100-400F depending on additives, grade of fuel, etc.

What Temperature Does Water Boil? Your Culinary Compass
The normal boiling point of water is 100 °C or 212 °F. Changes in elevation affect boiling point because they affect atmospheric pressure. The normal boiling point of water is 100 °C, 212 °F, or 373.1 K. The "normal" refers to sea level or an elevation of 0 meters or feet. But, the boiling point of water changes with elevation.
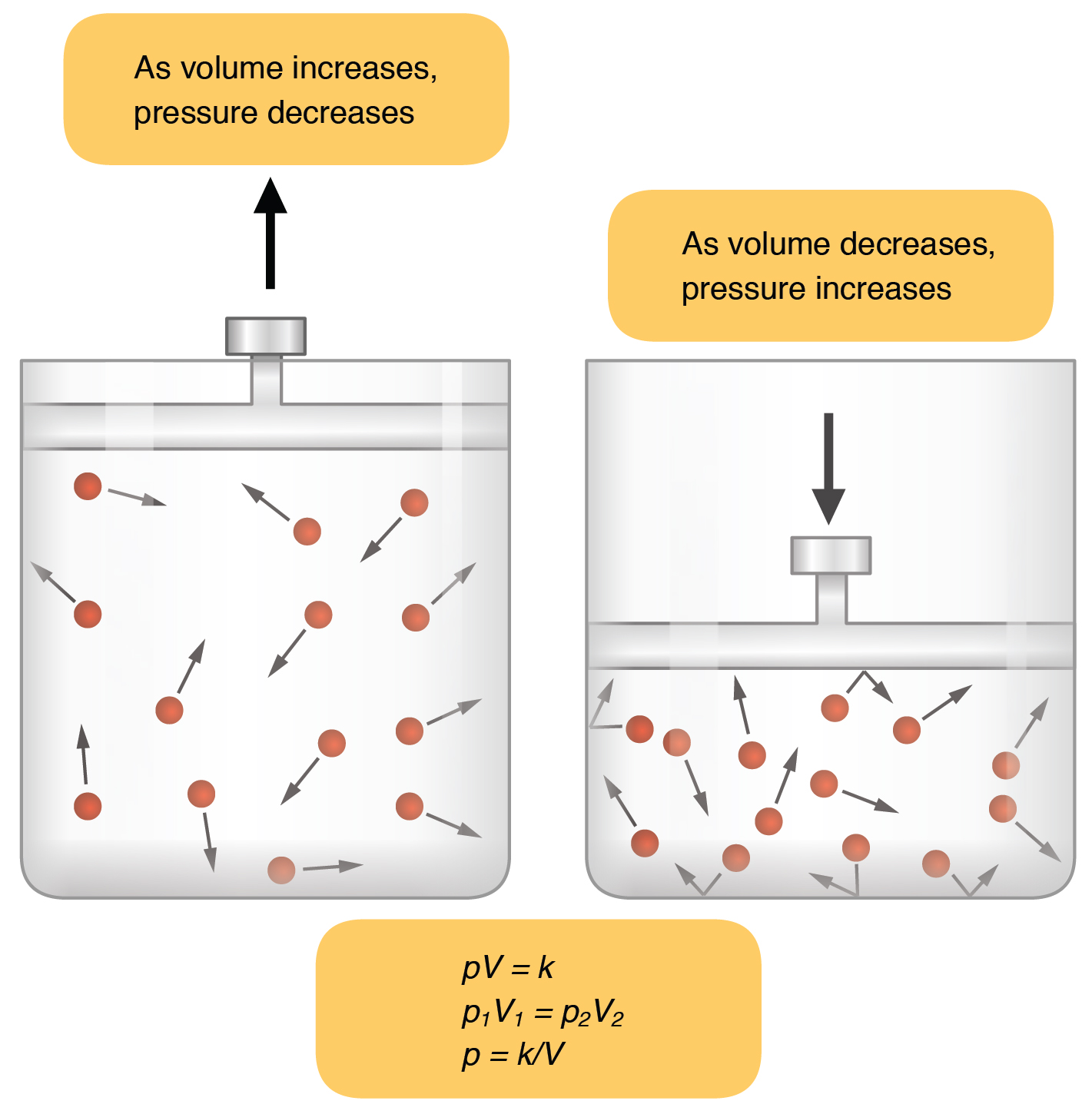
A sample of a gas has a volume of 2.0 liters at a pressure of 1.0
The temperature of a boiling liquid remains constant, even when more heat is added. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.The normal boiling point of a liquid is the temperature at which its vapor pressure is equal to one atmosphere (760 torr).

Introduction to Gas Freezing and Its Effects on Engines TemperatureAsk
Add to this, ethanol raises the RVP by an additional 1 psi (from 13.5 to 14.5). There is no question boiling can occur. In general, it can be said that ethanol will increase the likelihood of vapor lock by 10% just because it raises the RVP so much. It was the perfect storm of low boiling points and hot engines.
What temperature does alcohol boil? Quora
10.12: Boiling Point. When we heat a liquid until it boils, the bubbles that form inside the liquid consist of pure vapor. If the liquid is well stirred while boiling occurs, the vapor in the bubbles will be in equilibrium with the liquid and will have a pressure equal to the vapor pressure at the boiling temperature.
What Temperature Does 50 50 Antifreeze Freeze At Updated 2024
The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The figure below illustrates the boiling of liquid. Figure 13.9.2 13.9. 2: Comparison between evaporation and boiling. (Credit: Christopher Auyeung; Source: CK-12 Foundation; License: CC BY-NC 3.0 (opens in new window))

Boil water alert issued for part of West Bloomfield
The boiling point of a substance is the temperature at which it can change state from a liquid to a gas throughout the bulk of the liquid. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Some fuels and their boiling points at atmospheric pressure.

Boiling Point of Water What Temperature Does Water Boil?
Therefore the temperature of the liquid remains constant during boiling. For example, water will remain at 100ºC (at a pressure of 1 atm or 101.3 kPa) while boiling. A graph of temperature vs. time for water changing from a liquid to a gas, called a heating curve, shows a constant temperature as long as water is boiling.

Temperature sensing ingridscience.ca
Specifications define the temperatures at which. various percentages of the fuel are evaporated. Distillation limits include maximum temperatures that 10% is evaporated (50-70C), 50% is. evaporated (110-121C), 90% is evaporated (185-190C), and the final boiling point (225C). A minimum temperature for 50% evaporated (77C), and a maximum.

What is the lowest temperature setting on Masterbuilt electric smoker
Ethanol boils at 178.5 degrees F, while water boils at 212 degrees F. The trick in a carburetor is to make the fuel evaporate into fumes when atomized without lowering the boiling point.

High Altitude and Its Effect on Cooking
At the boiling point molecules anywhere in the liquid may be vaporized. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point at atmospheric pressure (14.7 psia, 1 bar absolute) for some common fluids and gases can be found from the.

An acidcatalyzed irreversible liquidphase reaction A B is carried out
miluutin. 9 years ago. In this paragraph of heat of vaporization I got a bit confused by these numbers: "Water's heat of vaporization depends on the temperature: it's around 540 cal/g at 100 °C (water's boiling point) and around 580 cal/g at 25 °C (room temperature)."

Real Gas vs Ideal Gas
The temperature of liquid nitrogen is −195.79 °C (77 K; −320 °F). Liquid nitrogen has many uses, but poses risks of frostbite, explosion, and suffocation if handled incorrectly. Liquid nitrogen is very cold! At room temperature and pressure, liquid nitrogen boils into nitrogen gas. It even looks like boiling water, except that it is.
What temperature does overheat protection kick in? Any way to check its
At standard atmospheric pressure, the propane boiling point is -44°F (-42°C). It will liquify when the temperature drops below -44°F and revert to its gas form if the temp reaches -44°F or higher. For instance, at -45 degrees F, this fuel will remain in a liquid state. One interesting fact: in most appliances, the propane can stay liquid.

How Much does Water Cool when Pouring?
Boiling points of common materials. Boiling point of water: 100 °C / 212 °F Boiling point of water (in Kelvin): 373.2 K Boiling point of ethanol: 78.37 °C / 173.1 °F Boiling point of methanol: 64.7 °C / 148.5 °F Boiling point of acetone: 56 °C / 132.8 °F Boiling point of alcohol: 78.37 °C / 173.1 °F Boiling point of nitrogen: -195.8.