
Exo vs Endo Products In The Diels Alder How To Tell Them Apart
10.7: Facial Selectivity. Page ID. Chris Schaller. College of Saint Benedict/Saint John's University. In addition to the consequences of endo- vs. exo- additions in the Diels Alder reaction, pericyclic reactions are subject to additional stereochemical constraints. In this section we will look at more issues of topology, or how the surfaces of.
Exo vs Endo Products In The Diels Alder How To Tell Them Apart
https://Leah4sci.com/DielsAlder presents: Diels Alder Reaction Part 3: Stereochemistry of the diene and dienophile and predicting Endo vs Exo Products📺Watch.
Exo And Endo Reactions
You have to remember 5-Endo-trig and 4-Exo-Dig as key points for Disfavored reactions. Figure 2.2.5. Baldwin cited several examples in support of these rules. Scientists soon attempted to validate the proposed rules. Steric and electronic factors appear to modify these conclusions. In most of the studies, the rules were generally applicable.
Endo vs Exo Why Are Endo Products Favored In DielsAlder Reactions?
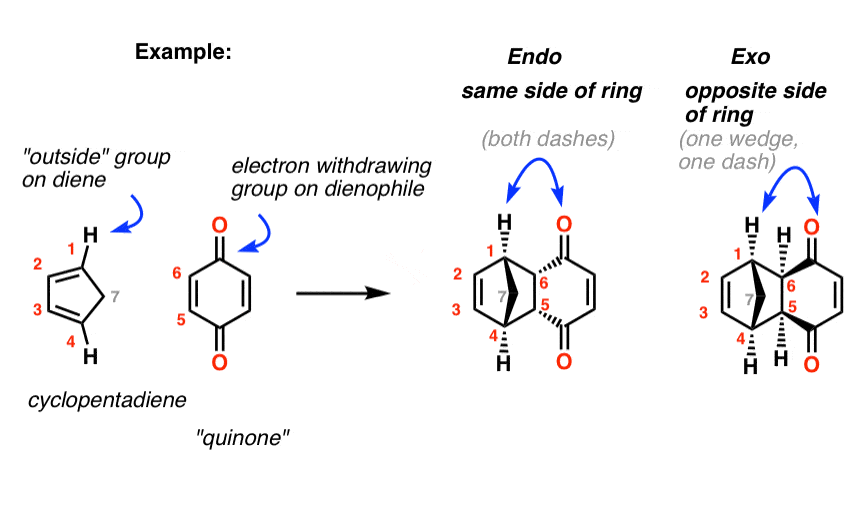
Exercise 10.5.1 10.5. 1. Draw the other stereisomers of the product formed from the reaction between furan and maleic anhydride. Answer. If we look at the molecule in this way, with the hydrogens highlighted on the ends of the diene and the dienophile, it may be easier to see the stereochmical relationships in the exo and endo products. In the.

38.03 Diastereomers from the DielsAlder Reaction Endo versus Exo
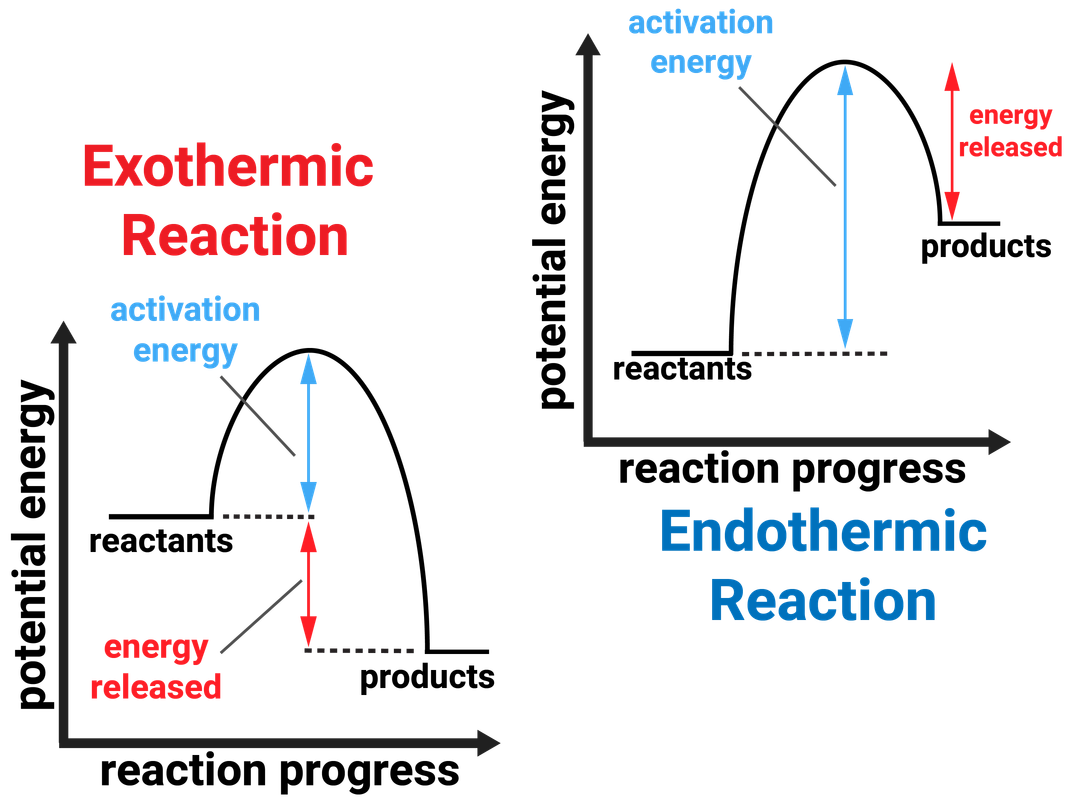
21 Great question! It turns out that the rate of formation of the "expected" endo product is actually ~500 times faster than the rate of formation of the exo product. However, the Diels-Alder is a reversible reaction. In this case, the exo product is thermodynamically favored over the endo product by about 1.9 kcal/mol 1.9 k c a l / m o l.
Exo vs Endo Products In The Diels Alder How To Tell Them Apart
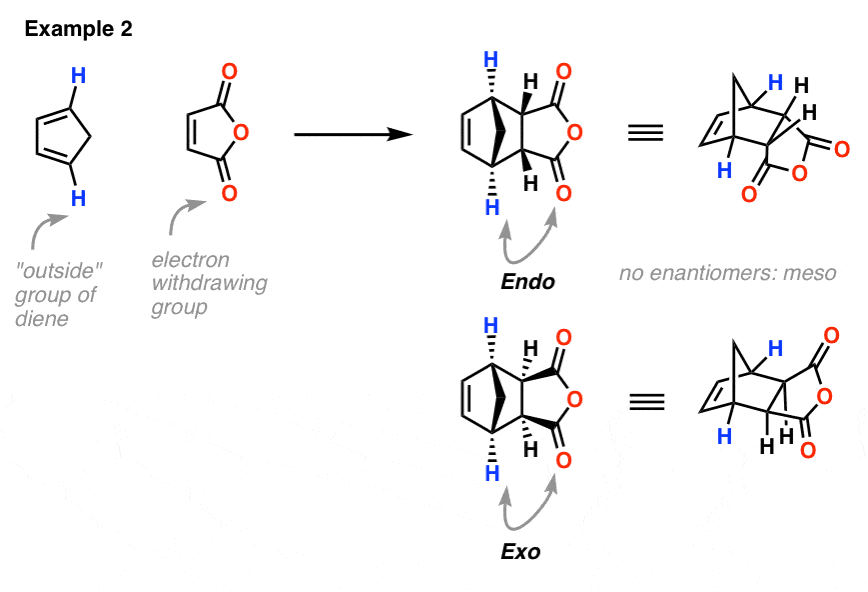
1. Exo and Endo Products in the Diels-Alder In this series of articles on the Diels-Alder reaction, we've seen that: the Diels-Alder reaction always involves the breakage of 3 pi bonds and the formation of 3 new bonds (2 sigma, one pi), resulting in the formation of a new six-membered ring. [ Intro]

PPT Endo vs. Exo Skeleton PowerPoint Presentation ID6738246
Water condensing as dew during the night. Endothermic. Dry ice subliming (changing from a solid directly to a gas). Exothermic. The wax in a candle burning. Exothermic. A match burning. Study with Quizlet and memorize flashcards containing terms like Exothermic, Endothermic, Exothermic and more.

Diels Alder Reaction Stereochemistry and Endo vs Exo Products YouTube
Endonuclease. In molecular biology, endonucleases are enzymes that cleave the phosphodiester bond within a polynucleotide chain (namely DNA or RNA ). Some, such as deoxyribonuclease I, cut DNA relatively nonspecifically (without regard to sequence), while many, typically called restriction endonucleases or restriction enzymes, cleave only at.
Exo vs Endo Products In The Diels Alder How To Tell Them Apart
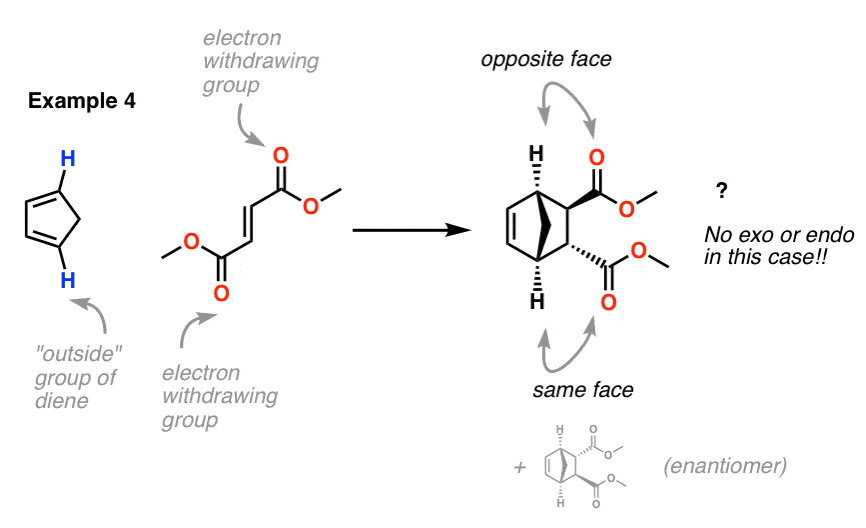
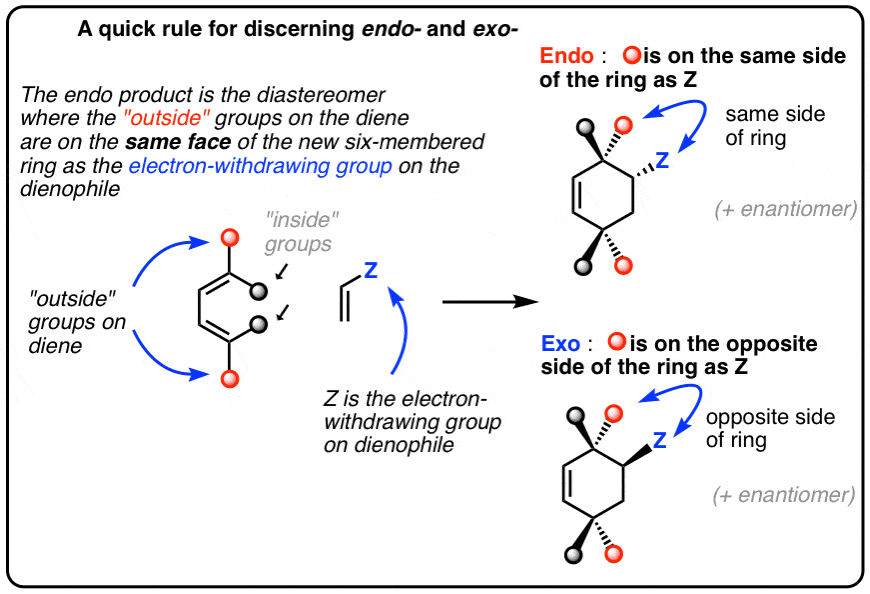
These two orientations lead to the formation of two products - endo and exo: In the endo product, the substituents of the dienophile are pointing towards the larger bridge, while in the exo isomer, they are pointing away from the larger bridge:

PPT Endo vs. Exo Skeleton PowerPoint Presentation, free download ID
Baldwin's rules Baldwin's rules in organic chemistry are a series of guidelines outlining the relative favorabilities of ring closure reactions in alicyclic compounds. They were first proposed by Jack Baldwin in 1976. [1] [2] Baldwin's rules discuss the relative rates of ring closures of these various types.
Exo vs Endo Products In The Diels Alder How To Tell Them Apart
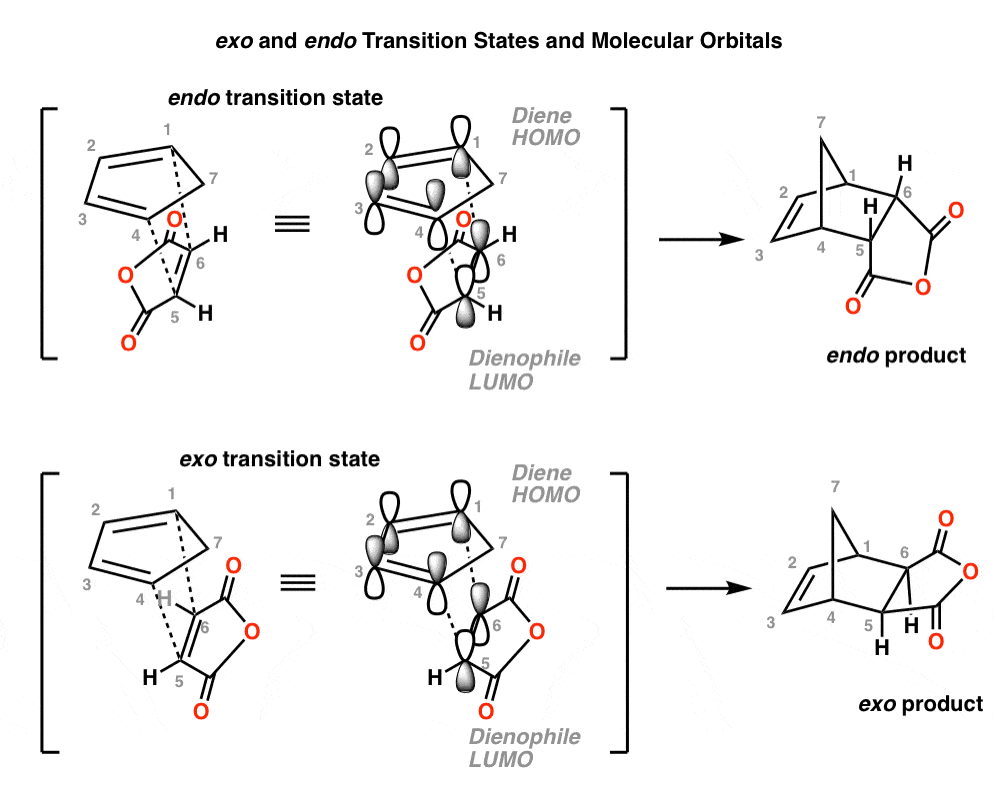
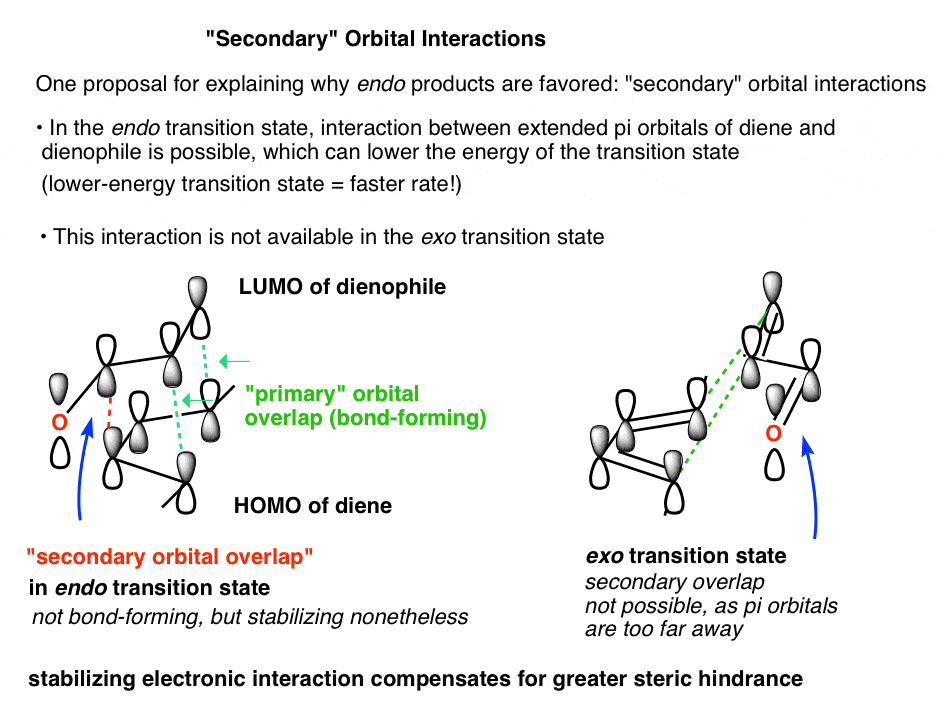
Even though this review has be over 50 years old, it still contains very useful information, so as the influence of Letis Acids on endo:exo selectivity. Endo and Exo transition states in the Diels-Alder reaction William C. Herdon and Lowell H. Hall Tetrahedron Lett. 1967, 8 (32), 3095-3100 DOI: 10.1016/S0040-4039(00)90922-5

Endo and Exo products of DielsAlder Reaction with Practice Problems
Endo vs. Exo Transition State: Generally, the endo transition state is favored. H H H H exo endo minor major Stereochemistry: In pericyclic reactions, the stereochemistry of the reactants is preserved in the product. Recall the cyclopropanation of alkenes by carbenes which is also a pericyclic reaction. R R R groups are trans in the reactant.
Exo vs Endo Products In The Diels Alder How To Tell Them Apart
1. Figure 7.3.1 7.3. 1: (A) Endothermic reaction. (B) Exothermic reaction. Endothermic Reaction: When 1mol 1 mol of calcium carbonate decomposes into 1 mol 1 mol of calcium oxide and 1 mol 1 mol of carbon dioxide, 177.8kJ 177.8 kJ of heat is absorbed. Because the heat is absorbed by the system, the 177.8kJ 177.8 kJ is written as a reactant.
Endo vs Exo Why Are Endo Products Favored In DielsAlder Reactions?
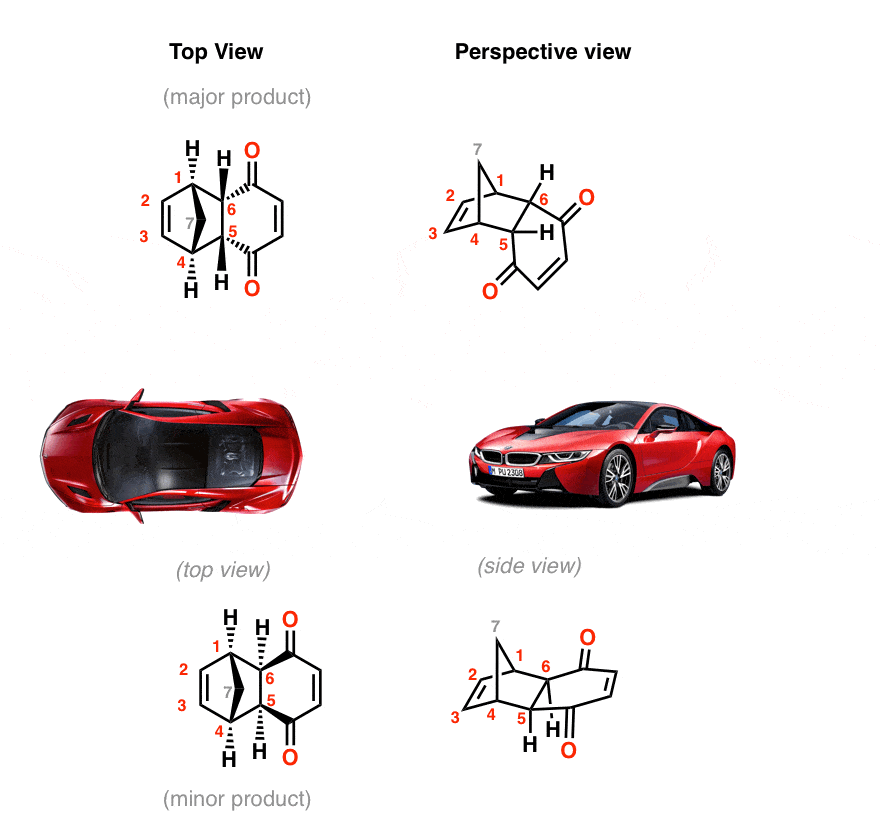
Th formation of exo vs endo is a case of kinetic vs. thermodynamic control. The exo product is more stable, but the activation energy for endo is lower, so the less stable endo product is formed faster. At lower temperatures, kinetic control prevails and the less stable endo isomer is the main product.
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)
Endothermic and Exothermic Chemical Reactions
One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. Let me first reveal the identity of your salts: Salt A is ammonium nitrate ( NH 4 NO 3 ) and Salt B is calcium chloride ( Ca Cl 2 )."

What is endo and exo configuration YouTube
Endo vs. Exo Transition State: Generally, the endo transition state is favored. H H H H exo endo minor major Stereochemistry: In pericyclic reactions, the stereochemistry of the reactants is preserved in the product. Recall the cylcopropanation of alkenes by carbenes which is also a pericyclic reaction.